
NOAA Fisheries Alaska Working On Program To Help Detect Shellfish Toxins Early
The following is courtesy of NOAA Fisheries Alaska:
Assessing Methods to Provide Early Warning of Paralytic Shellfish Toxin Events
April 09, 2024
NOAA Fisheries is working with partners to find the most accurate and effective methods to predict harmful algal blooms in Southeast Alaska.

Alexandrium catenella is the main species of algae that produces paralytic shellfish toxin in Southeast Alaska. Credit: University of Alaska Fairbanks/Courtney Hart.
Shellfish are a culturally and economically important resource in Southeast Alaska. Harmful algal blooms that produce paralytic shellfish toxins threaten safe subsistence harvest. They can lead to costly shutdowns for Alaska’s growing mariculture industry. To reduce public health risks and economic impacts, monitoring and predicting paralytic shellfish toxin events is crucial. Across Alaska’s vast and remote coastline, it is also highly challenging.
Paralytic shellfish toxins are produced by certain types of algae. The primary paralytic shellfish toxin-producing species in Southeast Alaska is Alexandrium catenella. Under certain conditions, algae populations can “bloom” to extremely high numbers.
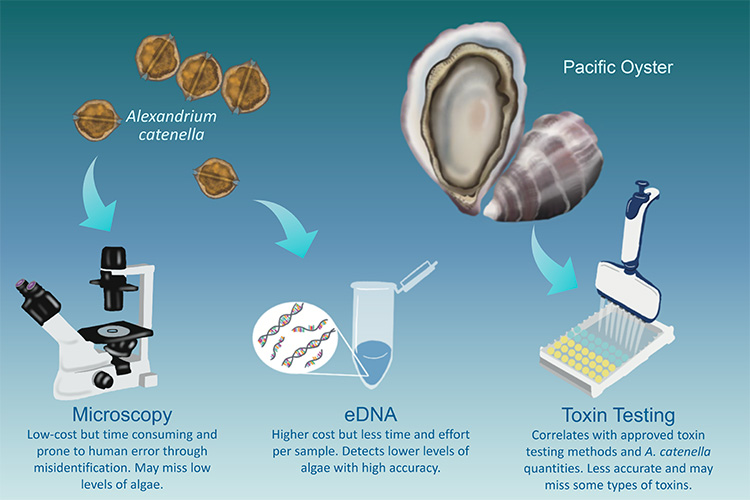
A new study sought to determine the best methods to provide timely warnings of these events for harvesters in Southeast Alaska. It assessed three methods to determine the most timely, accurate, and practical techniques to detect and quantify Alexandrium catenella and the toxins it produces.
The study found that eDNA is the best method for early and accurate detection, and should be incorporated into future monitoring efforts. However, further work is needed to refine eDNA analysis to provide timely and effective bloom warnings.
The study was led by NOAA Fisheries Alaska Fisheries Science Center in collaboration with:
- Alaska Sea Grant
- University of Alaska Fairbanks
- NOAA Hollings Scholarship program
- Fordham University
It was conducted in cooperation with Salty Lady Seafood Co. and in consultation with the Alaska Harmful Algal Bloom Network.
“There is a big need for widespread testing on Alaska beaches to inform subsistence and recreational harvesters. Understanding when and where to expect blooms could also help mariculture farmers plan ahead,” said study lead Juliana Cornett, NOAA Fisheries Alaska Fisheries Science Center. ”If we can detect harmful blooms before they become a threat, Alaska’s coastal communities and economy will greatly benefit.”

Paralytic Shellfish Toxins in Southeast Alaska
“Alexandrium catenella blooms can cause problems at lower levels than other toxin-producing algae. It is very important to be able to detect them before they reach those levels,” Cornett said.
When shellfish filter algae from these harmful algal blooms, the toxins accumulate in their tissues. If humans and animals eat shellfish with high levels of the toxin, they can become ill. At its worst, paralytic shellfish poisoning can lead to death.
Alaska has one of the highest rates of paralytic shellfish poisoning in the world.
“Alexandrium catenella blooms are a big threat here. Alaska is one of few places where there are still illnesses and occasional deaths from shellfish poisoning. And many of these cases occur in Southeast Alaska,” Cornett said.
Alaska’s vast coastline and the remoteness of many communities where subsistence or recreational shellfish harvest occurs makes regular monitoring for harmful algal blooms unfeasible. This has been a major factor in Alaska’s high rate of paralytic shellfish poisoning. Alaska has many community-led phytoplankton monitoring and shellfish testing programs. However, developing testing protocols that would allow for expanded routine monitoring could greatly reduce the risk of paralytic shellfish poisoning for Alaska communities.
Testing is required for commercial shellfish harvests in Alaska, including mariculture. But knowing what time of year to look out for harmful blooms, and what areas might be suitable for farms, could help minimize the economic impact of closures caused by paralytic shellfish toxins.
Researchers have compared methods for early detection of harmful blooms in other areas of the world. However, it is important to assess them in Southeast Alaska where unique ocean conditions can affect their efficacy.
“Some methods like microscope counts might not see Alexandrium catenella until they are at too high a level. With other methods we’re waiting until the toxins are in the shellfish—that’s too late,” Cornett said. “We were looking for an earlier detection method for subsistence and mariculture harvests. In particular, we were interested in testing environmental DNA to see if it could detect lower levels of A. catanella.”

Comparing Early Detection Methods for Alexandrium catenella and Paralytic Shellfish Toxins
The study compared the following detection methods:
- Microscopy—identifying and counting Alexandrium catenella cells collected from net tows and seawater samples
- eDNA analysis—detecting DNA shed by A. catenellainto seawater
- Toxin texting—quantifying paralytic shellfish toxins in oyster tissue samples (using one of two methods)
The research used Pacific oysters as a model shellfish. Pacific oysters are currently the most profitable species grown in the Alaskan mariculture industry. Many oyster farms are located in Southeast Alaska.

“Collaboration was key to the success of this research,” Cornett said. “This study wouldn’t have been possible without Meta Mesdag, the owner of Salty Lady Seafood Co. She provided transportation for us out to her farm site and provided all the oysters used in the study. She has collaborated with coauthors on this study for more than 6 years. Members of the Alaska Harmful Algal Bloom Network also provided great insight to guide the research.”

eDNA Detects Harmful Algae Early and Accurately
The study found that all three methods generally detected high levels of harmful algae and toxins. But eDNA detected much lower levels of Alexandrium catenella than the other methods.
eDNA samples also took less time and effort to process than microscopy. Identifying and counting algae via microscopy may take hours per sample by a trained analyst. Dozens of eDNA samples may be processed in a similar timeframe.
However, eDNA samples are typically processed in batches. There may be a significant lag before results are obtained. Processing eDNA samples one at a time would greatly increase the cost and effort per sample.
“eDNA is arguably the best method for early and accurate detection. However under current protocols it may not be the best suited to provide early warnings of blooms in an actionable timeframe for shellfish harvesters,” Cornett said.
Microscopy Is a Valuable Screening Tool
Microscopy is the cheapest method of phytoplankton monitoring and can provide a relatively quick count of a single sample at bloom levels. It can potentially be performed the day a sample is collected. Microscopy has been effectively used in monitoring programs led by members of the Alaska Harmful Algal Bloom network.
However, there were bloom days during the study period when microscopy failed to detect the algae. The study also found that some types of microscopy counts did not significantly relate to toxin values. Cornett says these findings raise concerns about the efficacy of microscopy alone as a paralytic shellfish toxin event warning. But, microscopy should not be dismissed as a useful tool in the harmful algal bloom toolkit.
“Considering the remote locations of many subsistence shellfish harvest areas in Alaska and lack of access to laboratory facilities, microscopy could still play a useful role as a screening tool.”
Future Directions: Developing Technology to Monitor for Harmful Algal Blooms in Alaska
The study found that eDNA offers many advantages over other methods, and should be incorporated into future monitoring efforts. However, Cornett notes that further work is needed to refine eDNA analysis to provide effective bloom warnings.
“With eDNA we could potentially detect the algae before a toxic event and provide an earlier warning of the presence of Alexandrium cells in the water. But there are still some unknowns. There were times when we detected elevated counts of Alexandrium in the water, but oysters weren’t toxic, and vice versa. It would be great to see further research to quantify how many cells of Alexandrium may lead to toxic oysters, and what other environmental or biological factors may influence toxin levels in both Alexandrium and oysters.”
Emphasis should also be placed on reducing the time and cost of eDNA analysis, and developing technology that could be used in more remote locations.
For routine monitoring, the researchers suggest using innovative, automated algae counting tools such as Imaging FlowCytobots. They could replace error-prone and time-consuming microscopy. These counts could be augmented with eDNA during bloom-prone months when its higher sensitivity would be most critical.
But even without further technological advances, eDNA could improve our ability to predict harmful blooms by advancing our understanding of what causes them.
“Even if eDNA is not currently the best ‘early warning’ method, it could be an incredibly valuable tool to help us learn to predict Alexandrium catenella blooms,” Cornett said. “The low limit of detection that eDNA offers could give us new insight into the drivers and dynamics of early bloom formation. That opens possibilities to predict blooms based on environmental conditions in the future.”



